Medical Device & Diagnostics
A HARMONIZED FRAMEWORK FOR MANAGING MEDICAL DEVICE QUALITY AND REGULATORY COMPLIANCE
The AssurX Medical Device Quality Management System: Aligning Quality Objectives with Compliance Requirements
The medical device market is full of opportunities to create life-saving products that diagnose and treat patients worldwide. AssurX medical device quality management system is designed to support manufacturers in marketing safe and effective medical devices. Additionally, it demonstrates compliance to meet those demands.
Device and diagnostics manufacturers use the AssurX EQMS to bridge the gap between aligning quality processes to improve product safety. They also maintain medical device compliance with applicable regulations.
TRUSTED BY
As an FDA-regulated company, Keystone Dental recognized the need to move beyond paper-based complaint handling processes that were hurting efficiency and increasing the complexity of reporting. By automating complaint management with AssurX, Keystone Dental was able to increase efficiency and accuracy while reducing the backlog of complaints and time to closure.
AssurX medical device quality management software helps enforce compliance with the following regulations and standards:
FEATURED SOLUTION
AssurX Electronic Medical Device (eMDR) solution provides a direct, closed-loop automated adverse event reporting without the requirement of any additional third-party tools or EDI systems. It enables full compliance with FDA CFR 21 Part 803 reporting guidelines for medical device manufacturers and device user facilities to report adverse events concerning device-related death or serious injury, or malfunction.
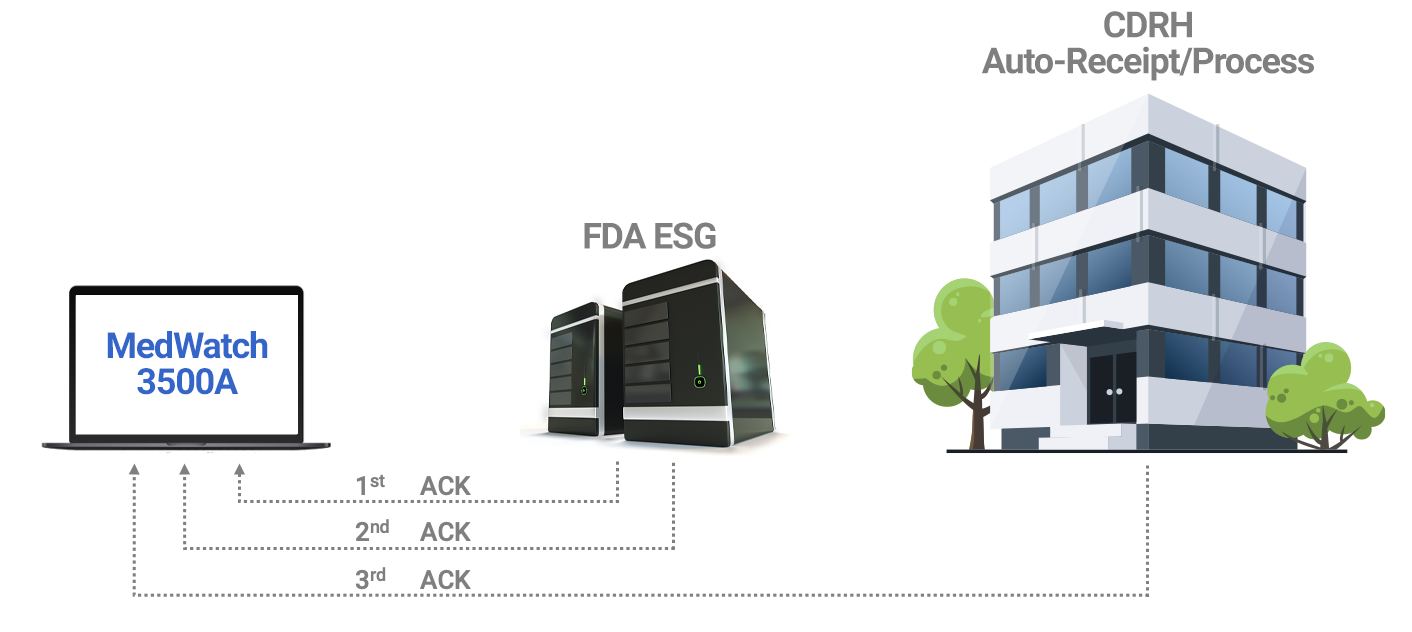
AssurX Medical Device Quality Management System Features
Manufacturers in the medical device industry and the diagnostics industry must maintain a laser focus on continuous quality improvement. Managing and controlling large volumes of documents, such as SOPs , training records, and quality manuals, is critical. A modern quality system using paper-based processes and siloed data is no longer viable.
Medical device QMS software facilitates compliance with stringent regulations by providing a framework for standardizing processes and maintaining proper documentation. Medical device compliance software automates document control, so teams use current and approved documents. This allows easier updates and audits for transparency and consistency.
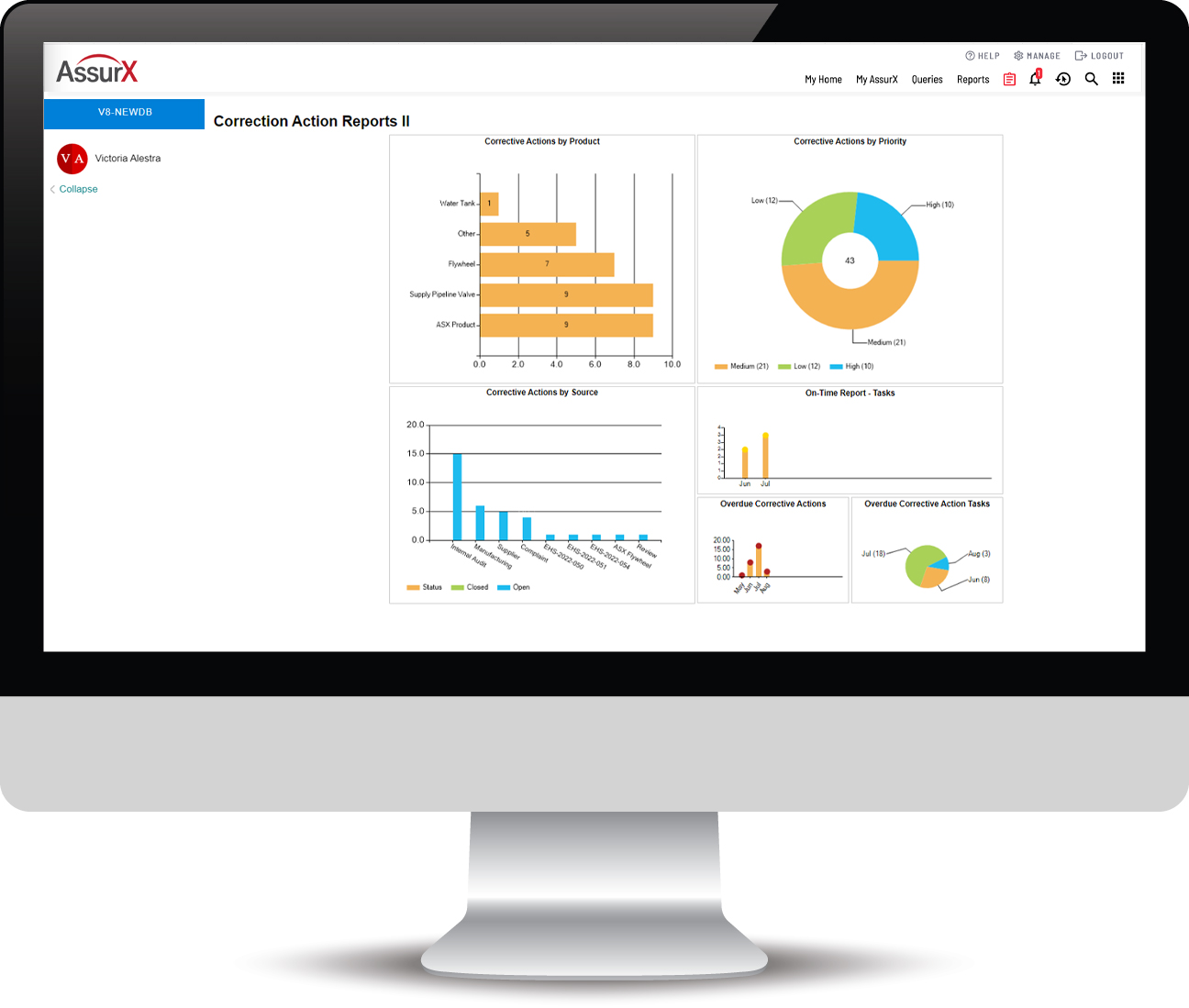
AssurX is built with robust features based on over 20 years of best practices, including:
Overcome Today’s Biggest Challenges for the Medical Device Industry
AssurX medical device quality management system accelerates your quality oversight to correct issues faster and implement preventive measures to prevent recurrence. Maintain a risk-based, proactive posture for efficient problem resolution.
Increased Efficiency
AssurX increases efficiency by integrating processes to close the loop on quality issues faster and more confidently. Launch CAPAs at any point in the quality chain. Our medical device QMS software can integrate risk management into all quality processes, ensuring a consistent approach to risk across the organization.
Quality Aligned with Compliance
AssurX makes it easier for your teams to participate in compliance efforts by incorporating regulatory requirements in your system. Configure the system to your organization’s unique medical device regulatory compliance demands. Our medical device compliance software helps you to be audit-ready. Its robust features provide comprehensive tracking and documentation capabilities.
Controls for HIPAA
Electronic PHI is accessible only to those who are allowed to see it. AssurX QMS software gives you the capability to set strict permission rights and shield unauthorized users from records that hold PHI.


Greater Control of Nonconformance Process
AssurX medical device manufacturing software provides a dynamic process for identifying, evaluating, documenting and disposition of non-conforming products as required per FDA 21 CFR 820 and ISO13485. Common terminology, failure codes, and escalation rules enable medical device and diagnostics manufacturers to identify and investigate the root cause of the issue diligently. They also demonstrate control of the non-conforming product, and use non-conformance data to help improve product quality at the design stage.
AssurX Complaint Management Software is a robust system designed to document, review, and report applicable adverse events to the FDA and other international bodies. Metrics & KPIs allow you to make informed decisions based on real-time data.
AssurX: Connected Quality and Validated Compliance
AssurX provides expert technical and program management oversight for customer deployments around the globe. We employ a structured implementation process, providing a consistent method for architecture design within our medical device quality management system.
Read the latest informative medical device industry posts >>>
AssurX: Quality & Compliance Systems for Every Enterprise
AssurX has been serving the medical device and diagnostics industry for more than two decades. Companies worldwide turn to us for help in reducing regulatory burden by automating quality processes while also demonstrating medical device regulatory compliance.
Our experts built an eQMS that can scale with your organization as it grows. Businesses need to shift and adapt frequently in the medical industry to stay competitive and our system allows that.
We can help you establish a new medical device quality management system or improve your existing system. Using unconnected ERP, PLM, and other enterprise applications? We can integrate them for a holistic approach to medical device regulatory compliance and optimal usage.





One Platform. Every Solution.
Easily integrate all your quality management and regulatory compliance processes into a single quality software system with AssurX. AssurX provides all core quality and compliance processes within the software system. This removes the need to budget for and install individual quality “modules.” Pre-configured workflows are available to you from day one with our quality management software.
Benefit from pre-configured workflows right from the start, ensuring a smooth transition to digital quality management. AssurX is an exceptional platform that facilitates transitioning from a labor-intensive, paper-based process to a streamlined electronic system. By adopting AssurX, you can embrace the enhanced organization that comes with digitizing documents and files.
With AssurX, enjoy the convenience of easily searchable data, allowing for quick retrieval and efficient information management, and saving valuable time. AssurX stands out as a user-friendly platform that allows for easy deployment, configuration, and integration with other software solutions.
First, implement the solutions you need now, then seamlessly connect more as you are ready. No other quality management system is easier to deploy, configure, and modify. Integrations with other software solutions make AssurX invaluable. Bid farewell to data silos and welcome a unified system for maximum efficiency.





























